As the demand for purified water continues to rise across various industries and applications, the distinction between distilled water and demineralised water emerges as a critical aspect of the water treatment landscape.
Choosing between these two purified water options can significantly impact outcomes in laboratories and industrial settings. These two seemingly similar water options differ in the purification process. If you need clarification about the right choice, this blog is for you.
Join us on a journey to understand the nuances, applications, and considerations shaping the decision-making process between distilled and demineralised water.
What is Demineralised Water?
Water, in its natural form, has numerous minerals and dissolved salts. Demineralisation is the process of removing these minerals and salts from water, thus reducing its mineral content. It involves the implementation of deionisation, where ion exchange resins are used to remove ions from the water selectively.

The goal is to produce free or nearly free water from dissolved minerals, salts, and other impurities. This purified water is often referred to as demineralised water or deionised water.
The primary minerals targeted in demineralisation are cations such as calcium (Ca2+), magnesium (Mg2+), sodium (Na+), and anions such as chloride (Cl-), sulfate (SO42-), and nitrate (NO3-).

The process involves passing water through ion exchange resin beds, where cations and anions are exchanged for hydrogen (H+) and hydroxide (OH-) ions. The ion exchange resins are periodically regenerated to maintain their effectiveness by removing captured ions and replacing them with fresh ones.
Check this video on the Demineralisation Process:
What is Demineralised water used for?
It is typically required for the following purposes: To prepare solutions and conduct experiments in laboratories, to prevent mineral deposits and scale formation in boilers and steam generators, for the production of semiconductors and electronic components, and for drug manufacturing and bioprocessing.
What is Distilled Water?
Distillation is a separation process of heating a liquid to generate vapour followed by condensation to produce a liquid. The process is based on the differences in boiling points of the components in a liquid mixture.
It is a widely used process for the purification of liquids and the separation of components in various industries, including chemistry, pharmaceuticals, petrochemicals, and beverage production.
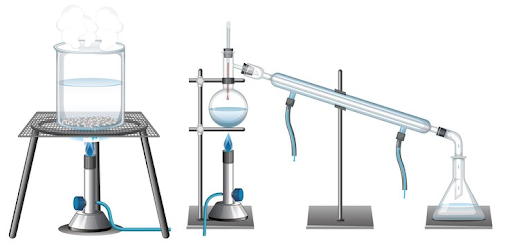
What are the Steps of Distillation?
1. Heating:
The liquid mixture, often called the “feed” or “charge,” is heated in a container called a distillation flask or still. When the distillation flask is heated, the component with the lower boiling point vaporises first.
2. Vaporisation:
The vaporised component rises through a column or tube called the distillation column. This distillation column can be packed with various materials or trays to enhance separation. The vaporisation process separates different components based on their boiling points.
3. Condensation:
As the vapour ascends the column, it reaches a cooler section called the condenser. The condenser cools the vapour, causing it to condense back into liquid form. This liquid is known as the distillate.
4. Collection:
The distillate is collected in a separate container called the receiver or receiving flask.
What are the types of Distillation?
Since distillation is a versatile technique, there are various types, such as simple distillation, fractional distillation, and steam distillation, each suitable for different purposes:
- Simple Distillation: Used for separating components with significantly different boiling points.
- Fractional Distillation: It is used when the components have closer boiling points.
Check this video about Fractional Distillation:
- Steam Distillation: Used for distilling heat-sensitive compounds, typically extracting essential oils from plants.
Check this video on Steam distillation:
Distillation serves as a method for purification and a means of concentrating or isolating specific components from a mixture. It’s a fundamental process in chemistry and industry for obtaining high-purity substances or separating complex mixtures into their components.
Distilled water is used for?
Distilled water is used for Solution preparation, experiments, chemical analyses, the production of intravenous (IV) fluids, autoclaves, and sterilisation in healthcare. Distilled water is recommended for topping off lead-acid batteries in vehicles.
Cooling systems of some cars and industrial machinery require distilled water to prevent mineral deposits and scale buildup in radiators and engine components. The formulation of cosmetics and toiletries and the production of electronic components need ultra-pure water. Distilled water replenishes water lost through electrolysis in lead-acid batteries, helping maintain proper electrolyte levels.
Distilled Water vs Demineralised Water
The two forms of highly purified water share the goal of removing impurities, yet they follow distinct methods, resulting in unique characteristics. Here is a comparison table highlighting 21 key differences to follow:
| Criteria | Distilled Water | Demineralised Water |
| Purification process | Distillation Process is used | Deionisation method is used |
| Purity | Purest form of water | Devoid of minerals but may contain microbial species |
| Contamination removal | Removes all kinds of impurities, including minerals, salts, and non-ionic contaminants | Primarily removes ionic impurities, leaving some non-ionic impurities |
| Ion Exchange | Does not involve ion exchange; instead, it relies on physical separation through condensation | Involves ion exchange, where ion exchange resins selectively remove ions from the water |
| Selective Ion Removal | Removes ions uniformly without selectivity | Ion exchange resins can be designed for selective removal of specific ions |
| Electricity Conductivity | Has very low electrical conductivity due to the removal of ions | It has low electrical conductivity but may be slightly higher than distilled water |
| Cost of Production | The distillation process can be energy-intensive, affecting the cost of production | Deionisation may have lower production costs compared to distillation |
| Residue Formation | Leaves no residue after evaporation, as most impurities are removed | May leave some residue, depending on the non-ionic impurities present |
| Effect on Equipment | Generally less likely to cause scale buildup or corrosion in equipment | May contain some ions that could contribute to scale or corrosion under certain conditions |
| Microbial Content | Eliminates all microbes | Microbes are not eliminated |
| Chemical Composition | Only H2O with minimal impurities | Contains H2O and may have some non-ionic impurities |
| Taste | Typically Tasteless | It may retain a slight taste from non-ionic impurities |
| Water Hardness | Completely free from water hardness, as the minerals causing hardness are removed | Low in water hardness, but some hardness may still be present based on the effectiveness of the deionisation process |
| pH Levels | Typically has a neutral pH of 7 | pH can be adjusted depending on the source water and treatment process |
| Storage stability | Generally stable during storage, with minimal changes in composition | Stable but may be influenced by the stability of ion exchange resins |
| Scale Formation | Rarely forms scale due to the absence of minerals | May form scale under certain conditions, as not all minerals are removed |
| Usage in Steam generation | Preferred for steam generation to prevent scale buildup in boilers | It is also suitable for steam generation but may require additional treatment to minimise scale formation |
| Automotive Applications | Used in automotive cooling systems to avoid mineral deposits | It can also be used in automotive applications but may require monitoring for specific ionic content |
| Biological Impact | Generally safe for biological systems and processes | It is also safe, but the impact may depend on the particular non-ionic impurities present |
| Environmental Impact | The distillation process may have a higher environmental impact due to energy consumption. | Deionisation is more environmentally friendly in terms of energy use. |
| Health Concerns | It is safe for consumption but is not recommended as it lacks the necessary minerals. | It is unsafe for consumption as it may contain some microbes and non-ionic impurities. |
Conclusion
In conclusion, choosing between distilled water and demineralised water ultimately depends on the specific requirements of your intended use. Distilled water, with its comprehensive removal of impurities, is ideal for applications that demand the highest purity standards, such as laboratories and specific medical processes.
On the other hand, demineralised water, while highly pure, may retain some non-ionic impurities, making it suitable for various industrial uses. Always consider the intended application and the level of purity required to ensure optimal performance and effectiveness.
FAQs
1. What is the purification process?
A: Water purification refers to the removal of any unwanted material from the water. The methods used for purification generally include boiling water, distillation, demineralisation, and filtration.
2. Can You Drink Demineralized Water?
A: No, this water lacks the necessary minerals and may contain other impurities like microbes or non-ionic impurities.
3. Can I use demineralised water instead of distilled water?
A: The demineralisation process does not remove any suspended particles, organic compounds, or microbes as effectively as the distillation process. Distillation produces pristine water; hence, it may not be replaced with demineralised water, which requires ultra-pure water.
Key Takeaways
- Distilled water is the purest form of water in comparison to Demineralised water.
- The four basic distillation steps are Heating, Vaporisation, Condensation, and Colection.
- The types of distillation include simple, fractional, and steam.
- Purifying water using demineralisation is a cost-effective process.
- Demineralisation involves the implementation of ion exchange resins to remove ionic impurities in the water.
- Demineralised water is unsafe for consumption as it can contain organic impurities, bacteria, and viruses.
- Distilled water is safe for consumption but not recommended for long-term use as it needs the necessary minerals.






